Rapid increase in environmental pollution due to a fast growing population has become a concern, which requires serious political and scientific attention. Artificial micromotors hold a great potential to remove or destroy toxic chemicals from industrial wastewaters.
What Are Artificial Micromotors?
Artificial micromotors are tiny devices with dimensions several orders less than the thickness of a human hair, capable of undergoing autonomous motion in liquid environments. Unlike a bacterium which makes use of its flagella for motility, artificial micromotors can be actuated by either an external magnetic field, upon irradiation of ultraviolet or infrared light, or by means of a chemical fuel.
The type of micromotors which require a chemical fuel for their motion, are termed as catalytic micromotors. Such micromotors are composed of a catalytic materials which can decompose a fuel (typically hydrogen peroxide) into water and oxygen gas bubbles. The generation and accumulation of gas bubbles at the solid-liquid interface, facilitated by the addition of a surfactant, provides a driving force to the particle in the opposite direction, resulting in a net directional motion.
Why the Need For Micromotors?
The use of chemicals and organic dyestuff in the industrial manufacturing processes is essential and unavoidable. However, uncontrolled release of these materials into rivers and lakes in the form of wastewater poses enormous threat to environment. Most of these materials are non-biodegradable, which means that bacteria cannot degrade such chemicals. Hence, other approaches have to be implemented for the degradation or removal of these chemicals. The most commonly employed methods for the removal of these pollutants are adsorption, coagulation, oxidative degradation or photocatalytic degradation. These methods require external means of mixing to increase the rate of chemical reactions involved in the degradation process. However, this cannot be very convenient and requires a mechanical agitator; adding up to the overall cost of the operation.
In addition to generating oxygen gas bubbles, a catalytic micromotor generates highly reactive chemical species, called radicals, which can readily destroy toxic chemicals. Furthermore, these tiny devices can simultaneously act as microscale mixers, causing chaotic mixing in liquids. This synergistic effect can lead to higher water cleaning efficiency. However, most widely explored catalytic materials are either too precious (platinum) or very toxic (silver) to aquatic life.
Micromotors In Action
Our group at the Department of Chemistry, University of Eastern Finland, has recently demonstrated a proof-of-concept study about the application of manganese oxide (MnO2) based micromotors for cleaning polluted water. Manganese oxide is an environment-friendly, biocompatible catalytic material which can decompose H2O2 in a way similar to platinum or silver. Being an abundant part of earth’s crust, MnO2 is a very low cost material and can also be synthesized in the laboratory. We noticed that the MnO2 micromotors synthesized in the laboratory undergo rapid degradation with a lifetime of only a few minutes.1
In order to be applicable for water cleaning tasks, the micromotors should possess sufficiently longer operational life to complete a cleaning cycle. Interestingly, commercially available MnO2 microparticles fulfilled this criterion. Commercial MnO2 microparticles could undergo motion for extended periods of time, reaching speeds up to 1000 μm s-1 in the presence of 10% H2O2 fuel.2 These speeds correspond to 100 body lengths s-1; roughly 10 times higher than the swimming speed of a bacterium. The MnO2 micromotors could move even in the presence of 0.5% fuel, at an average speed of 152 μm s-1.
An additional feature of MnO2 micromotors is their sustained motion even in the presence of salt rich environments; i.e. the conditions which severly hamper the performance of platinum based micromotors. Only a slight decrease in the speeds could be noticed. The motion of a MnO2 micromotor can be tracked by an optical microscope, as follows.
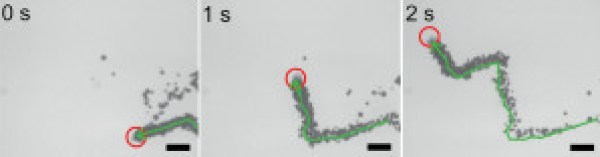
The Dye Removal Process
The process of dye removal involves two mechanisms; (i) catalytic degradation (CD), and (ii) adsorptive bubble separation (ABS). In the first mechanism, the catalytic surface of the micromotors decomposes H2O2 fuel into oxygen gas, water and radicals. The radicals instantly react with dye molecules and cause them to break down into harmless byproducts.

In the second mechanism, the excess dye molecules electrostatically adsorb on the gas bubbles formed in the presence of a surfactant. As the micromotors continue to produce excessive amount of bubbles, a column of bubbles is formed at the top of solution as seen below.

The resulting column of bubbles can be separated and the adsorbed dye can be recovered for reuse or disposal. This dual effect, i.e. CD and ABS, leads to over 90% of water decolorization in only an hour, without the need of external means of mechanical agitation. The speeds of micromotors are dependent on the concentration for fuel used. We analyzed speeds of micromotors in the fuel concentrations of 0.5% – 10%. The aim was to find an optimum fuel level which could provide efficient dye removal. A concentration of 5% was chosen for remediation experiments which resulted in an average micromotor speed up to 600 μm s-1. The final concentration of the surfactant was chosen to be 0.1%, which was sufficient to produce fast motion and enormous quantity of bubbles. Only 5 mg of the MnO2 microparticles were added to 10 mL of contaminated water, to function as micromotors. The shape of a typical MnO2 microparticle is shown below.

These experiments suggest a great potential of autonomously moving artificial micromotors to do remediation tasks at remote field locations and difficult to reach areas, such as lakes, sewage pipes etc. The efficient catalytic activity, ease of availability, abundance and environment friendliness of MnO2 are promising features to function as micromotors for water remediation tasks. Future work is focused to employ MnO2 micromotors for the removal of toxic heavy metals and phenolic compounds from polluted waters.
References:
1. Safdar, M.; Wani, O. M.; Jänis, J. Manganese Oxide Based Chemically Powered Micromotors. ACS Appl. Mater. Interfaces 2015, 7, 25580−25585.
2. Wani, O. M.; Safdar, M.; Kinnunen, N.; Jänis, J. Dual Effect of Manganese Oxide Micromotors: Catalytic Degradation and Adsorptive Bubble Separation of Organic Pollutants. Chem. Eur. J. 2015, 21, 1–5.
Learn more about PreScouter at www.prescouter.com.







